Playing with dirt...For SCIENCE!!!
|
My niece has a science fair in about a week and we decided to brainstorm some ideas since somebody is already working on the solar oven she wanted to build. So I pitched a few ideas; windmill, lemon battery, potato battery, and a few dumb ideas also which she shot down - and then it hit me. A few months back I saw a video like this one where a guy built a battery outta dirt. I showed my niece the video and she decided that was the project she wanted to do. The experiment would be to see what liquid would make the battery better.
So I am going to document a bit of the project here, though the idea is still forming. A few things we decided to test for the science fair project: 1. What is the best electrolyte? a. Water b. vinegar c. salt water d. lemon juice (the lemon battery rears it's Stewie Griffin like head once more!) 2. Which of the above electrolytes will light up a LED the brightest. |
We will also measure the voltage of the battery with and without a load, and then will test the current through the LED (which I forgot to do in the first test run). We actually went ahead and did a test just to make sure it worked and yeah it does, but there were a few things to sort out. The electrodes are very poor, just galvanized roofing nails (I think a small piece of galvanized sheet would be better). The copper conductor is also just a bit of solder braid so that needs improvement. It does work however. Below is a "dry" run, the soil was just slightly damp, enough to clump together. The output was 6.27V unloaded and it would not even tickle the LED enough to do anything.
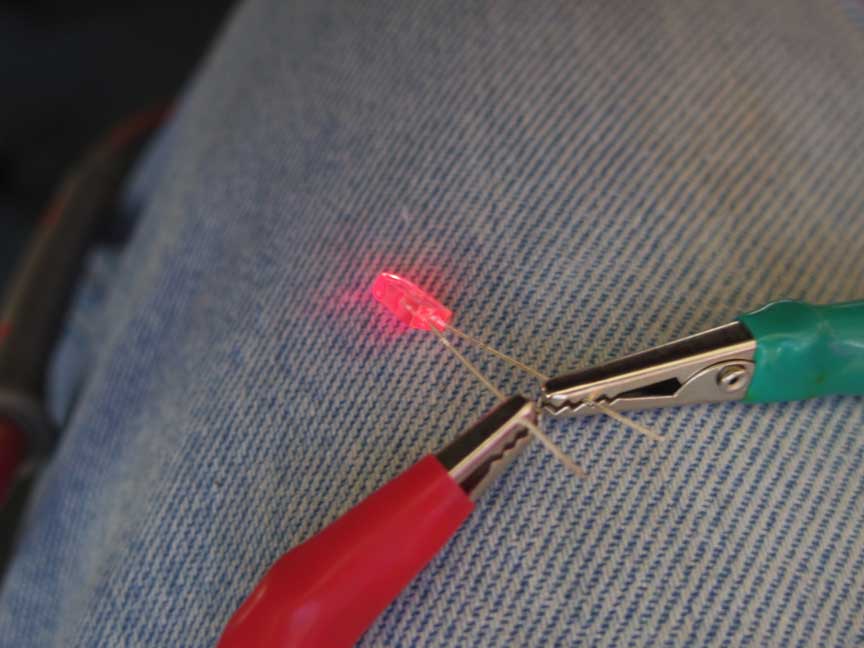
Next up, we added some tap water to the tray, making sure that each cube holder was not "shorted" to the next one with stray dirt and water. This time the unloaded voltage was up to 9.34V (an increase of about 3V) and the LED did manage to squeak out a tiny glow. The voltage drop over the LED was 1.43V and it was measuring a whopping 0.07mA.
So that's where we stopped since we want to improve the battery design with bigger plates, after that we will start testing different electrolytes.
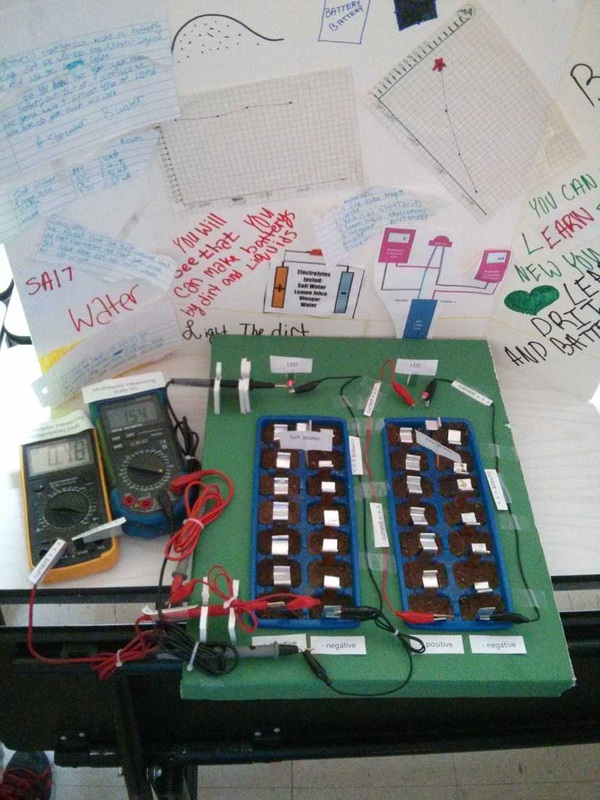
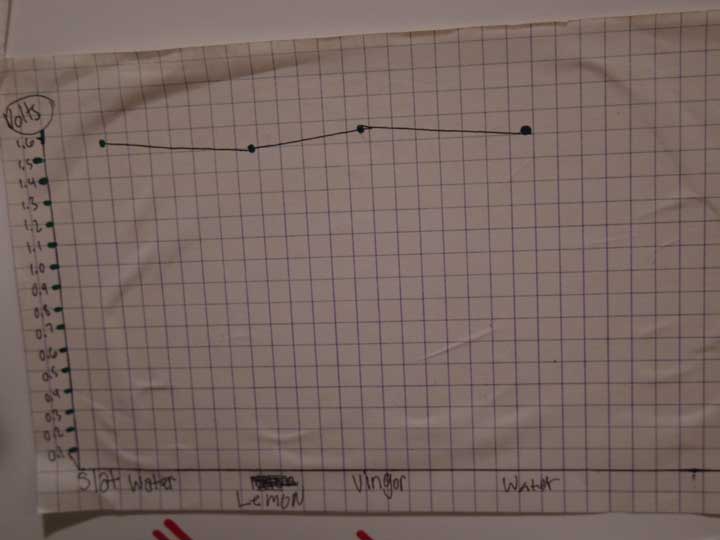
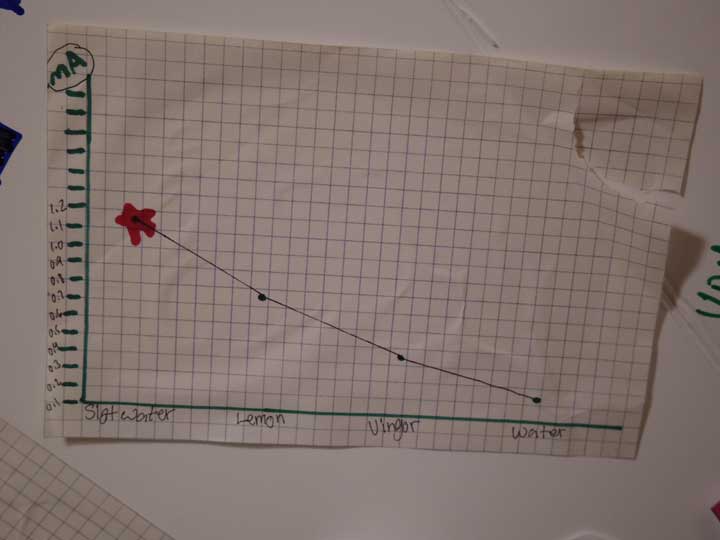
UPDATE: We had an idea for a better plate design, so we picked up some copper and galvanized sheet and cut them into strips, then rolled one end together and formed a clip which created the plates and their connection. After that we tried 4 trays filled with dirt and different electrolytes (salt water, lemon juice, vinegar and tap water). The tests showed that salt water is the best by a long shot. It was also interesting that even after almost 2 days, the batteries with the salt water and the water were still lighting a LED (dimly) - in fact the picture below showing the science fair display was taken after almost 2 days of running without additional water. We did not keep the vinegar or lemon juice batteries going after the initial tests, and kept the water and salt water batteries around for the science fair display.
UPDATE: We had an idea for a better plate design, so we picked up some copper and galvanized sheet and cut them into strips, then rolled one end together and formed a clip which created the plates and their connection. After that we tried 4 trays filled with dirt and different electrolytes (salt water, lemon juice, vinegar and tap water). The tests showed that salt water is the best by a long shot. It was also interesting that even after almost 2 days, the batteries with the salt water and the water were still lighting a LED (dimly) - in fact the picture below showing the science fair display was taken after almost 2 days of running without additional water. We did not keep the vinegar or lemon juice batteries going after the initial tests, and kept the water and salt water batteries around for the science fair display.